Tokisetso ea barium
Tokisetso ea indasteri eabarium ea tšepee kenyelletsa mehato e 'meli: ho lokisoa ha barium oxide le ho lokisoa ha metallic barium ka ho fokotsa mocheso oa tšepe (aluminothermic reduction).
| Sehlahisoa | Barium | ||
| Nomoro ea CAS | 7647-17-8 | ||
| Nomoro ea sehlopha. | 16121606 | Bongata: | 100.00kg |
| Letsatsi la tlhahiso: | Tšitoe,16,2016 | Letsatsi la teko: | Tšitoe,16,2016 |
| Ntho ea teko w/% | Liphetho | Ntho ea teko w/% | Liphetho |
| Ba | >99.92% | Sb | <0.0005 |
| Be | <0.0005 | Ca | 0.015 |
| Na | <0.001 | Sr | 0.045 |
| Mg | 0.0013 | Ti | <0.0005 |
| Al | 0.017 | Cr | <0.0005 |
| Si | 0.0015 | Mn | 0.0015 |
| K | <0.001 | Fe | <0.001 |
| As | <0.001 | Ni | <0.0005 |
| Sn | <0.0005 | Cu | <0.0005 |
| Tekanyetso ea Teko | E-ba, Na le likarolo tse ling tsa 16: ICP-MS Ca, Sr: ICP-AES Ba: TC-TIC | ||
| Qetello: | Latela maemo a khoebo | ||

(1) Tokisetso ea barium oxide
Ore ea boleng bo holimo ea barite e tlameha ho qala ka ho khethoa ka letsoho le ho phaphamala, ebe tšepe le silicon li tlosoa ho fumana concentrate e nang le barium sulfate e fetang 96%. Phofo ea ore e nang le boholo ba likaroloana tse ka tlase ho 20 mesh e kopantsoe le phofo ea mashala kapa petroleum coke ka tekanyo ea boima ba 4: 1, 'me e halikiloe ka 1100 ℃ ka sebōping sa reverberatory. Barium sulfate e fokotsehile ho barium sulfide (eo hangata e tsejoang e le "black ash"), 'me tharollo e fumanoeng ea barium sulfide e tšeloa ka metsi a chesang. E le ho fetola barium sulfide hore e be barium carbonate precipitation, sodium carbonate kapa carbon dioxide e hloka ho kenngoa ho barium sulfide aqueous solution. Barium oxide e ka fumanoa ka ho kopanya barium carbonate le carbon powder le ho e calcining ka holimo ho 800 ℃. Re lokela ho hlokomela hore barium oxide e oxidized ho etsa barium peroxide ho 500-700 ℃, le barium peroxide e ka senyeha ho etsa barium oxide ho 700-800 ℃. Ka hona, e le ho qoba tlhahiso ea peroxide ea barium, sehlahisoa sa calcined se hloka ho pholile kapa ho tima tlas'a tšireletso ea khase ea inert.
(2) Mokhoa oa ho fokotsa aluminothermic ho hlahisa barium ea tšepe
Ka lebaka la metsoako e fapaneng, ho na le karabelo e 'meli ea aluminium e fokotsang barium oxide:
6BaO+2Al→3BaO•Al2O3+3Ba↑
Kapa: 4BaO+2Al→BaO•Al2O3+3Ba↑
Ho 1000-1200 ℃, liketso tsena tse peli li hlahisa barium e nyenyane haholo, kahoo pompo ea vacuum ea hlokahala ho tsoela pele ho fetisetsa mouoane oa barium ho tloha sebakeng sa karabelo ho ea sebakeng sa condensation e le hore karabelo e ka tsoela pele ho ea ka ho le letona. Masala a ka mor'a ho arabela a na le chefo 'me a hloka ho phekoloa pele a ka lahloa.
Tokisetso ea metsoako e tloaelehileng ea barium
(1) Mokhoa oa ho itokisa oa barium carbonate
① Mokhoa oa carbonization
Mokhoa oa carbonization haholo-holo o kenyelletsa ho kopanya barite le mashala ka tekanyo e itseng, ho li pshatla ka sebōping sa rotary le calcining le ho li fokotsa ho 1100-1200 ℃ ho fumana sulfide ea barium sulfide. Carbon dioxide e kenngoa ka har'a tharollo ea barium sulfide bakeng sa carbonization, 'me karabelo ke e latelang:
BaS+CO2+H2O=BaCO3+H2S
Barium carbonate slurry e fumanoeng e hlatsuoa, e hlatsuoa ebe e tlhotloa, ebe e omisoa le ho siloa ka 300 ℃ ho fumana sehlahisoa se felileng sa barium carbonate. Mokhoa ona o bonolo ts'ebetsong ebile o theko e tlaase, kahoo o amoheloa ke bahlahisi ba bangata.
② Mokhoa oa ho qhibiliha habeli
Barium sulfide le ammonium carbonate li na le karabelo ea ho bola habeli, 'me karabelo e tjena:
BaS+(NH4)2CO3=BaCO3+(NH4)2S
Kapa barium chloride e arabela ka potassium carbonate, 'me karabelo e tjena:
BaCl2+K2CO3=BaCO3+2KCl
Sehlahisoa se fumanoeng ho tloha karabelo se hlatsuoa, se tlhotliloeng, se omisitsoe, joalo-joalo ho fumana sehlahisoa se felileng sa barium carbonate.
③ mokhoa oa Barium carbonate
Barium carbonate phofo e ts'oaroa ka letsoai la ammonium ho hlahisa letsoai la barium e qhibilihang, 'me ammonium carbonate e sebelisoa hape. Soluble barium letsoai le eketsoa ho ammonium carbonate ho etsa precipitate refined barium carbonate, e tlhotliloeng le ho omisoa ho etsa sehlahisoa se felileng. Ho feta moo, joala ba 'm'e bo fumanoeng bo ka sebelisoa hape. Karabo e tjena:
BaCO3+2HCl=BaCl2+H2O+CO2
BaCl2+2NH4OH=Ba(OH)2+2NH4Cl
Ba(OH)2+CO2=BaCO3+H2O
(2) Mokhoa oa ho itokisa oa titanate ea barium
① Mokhoa o tiileng oa mohato
Barium titanate e ka fumanoa ka calcining barium carbonate le titanium dioxide, 'me lisebelisoa leha e le life tse ling li ka kenngoa ho eona. Karabo e tjena:
TiO2 + BaCO3 = BaTiO3 + CO2↑
② Mokhoa oa ho nyenyefatsa
Barium chloride le titanium tetrachloride li tsoakoa 'me li qhibiliha ka tekanyo e lekanang, e futhumatsoa ho 70 ° C, ebe oxalic acid e kenngoa ka tsela e fokolang ho fumana hydrated barium titanyl oxalate [BaTiO(C2O4)2•4H2O] precipitate, e hlatsuoang, e omisitsoeng, ebe e fumana pyrolyzed ho fumana pyrolyzed. Karabo e tjena:
BaCl2 + TiCl4 + 2H2C2O4 + 5H2O = BaTiO(C2O4)2•4H2O↓ + 6HCl
BaTiO(C2O4)2•4H2O = BaTiO3 + 2CO2↑ + 2CO↑ + 4H2O
Ka mor'a ho otla metatanic acid, tharollo ea barium chloride e eketsoa, 'me joale ammonium carbonate e kenngoa ka tlas'a ho sisinyeha ho hlahisa coprecipitate ea barium carbonate le metatitanic acid, e leng calcined ho fumana sehlahisoa. Karabo e tjena:
BaCl2 + (NH4)2CO3 = BaCO3 + 2NH4Cl
H2TiO3 + BaCO3 = BaTiO3 + CO2↑ + H2O
(3) Tokisetso ea barium chloride
Ts'ebetso ea tlhahiso ea barium chloride haholo-holo e kenyelletsa mokhoa oa hydrochloric acid, mokhoa oa barium carbonate, mokhoa oa calcium chloride le mokhoa oa magnesium chloride ho latela mekhoa e fapaneng kapa lisebelisoa tse tala.
① Mokhoa oa Hydrochloric acid. Ha barium sulfide e phekoloa ka hydrochloric acid, karabelo e kholo ke:
BaS+2HCI=BaCl2+H2S↑+Q
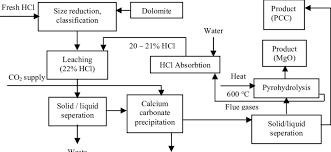
②Mokhoa oa Barium carbonate. E entsoe ka barium carbonate (barium carbonate) joalo ka lisebelisoa tse tala, karabelo ea mantlha ke:
BaCO3+2HCI=BaCl2+CO2↑+H2O
③Mokhoa oa carbonization

Liphello tsa barium bophelong ba motho
Barium e ama bophelo bo botle joang?
Barium ha se ntho ea bohlokoa bakeng sa 'mele oa motho, empa e na le tšusumetso e kholo bophelong ba motho. Barium e ka 'na ea pepesehela barium nakong ea merafo ea barium, ho qhibiliha, ho etsa tlhahiso le tšebeliso ea metsoako ea barium. Barium le metsoako ea eona e ka kena 'meleng ka tsela ea ho hema, tšilo ea lijo le letlalo le senyehileng. Chefo ea barium mosebetsing e bakoa haholo-holo ke ho hema ho phefumoloha, ho hlahang likotsing nakong ea tlhahiso le tšebeliso; chefo ea barium e sa sebetseng haholo-holo e bakoa ke ho kenngoa ha tšilo ea lijo, haholo-holo e bakoang ke ho kenngoa ka phoso; Metsi a qhibilihang a barium a ka monngoa ka letlalo le lemetseng. Chefo e matla ea barium hangata e bakoa ke ho noa ka phoso.
Tšebeliso ea bongaka
(1) Radiography ea lijo tsa Barium
Barium meal radiography, eo hape e tsejoang e le digestive tract barium radiography, ke mokhoa oa tlhahlobo o sebelisang barium sulfate e le ntho e fapaneng ho bontša hore na ho na le maqeba ka har'a tšilo ea lijo tlas'a mahlaseli a X-ray. Barium meal radiography ke ho kenngoa ka molomo ha metsoako e fapaneng, 'me moriana oa barium sulfate o sebelisoang e le motsoako o fapaneng ha o kenelle metsing le lipids' me o ke ke oa kenngoa ke mucosa ea mala, kahoo ha e le hantle ha e chefo ho batho.

Ho ea ka litlhoko tsa tlhahlobo ea bongaka le phekolo, radiography ea lijo tsa gastrointestinal barium e ka aroloa ka lijo tse ka holimo tsa gastrointestinal barium, lijo tsohle tsa gastrointestinal barium, colon barium enema le tlhahlobo e nyenyane ea intestinal barium enema.
Chefo ea Barium
Litsela tsa ho pepeseha
Barium e ka pepesetsoabariumnakong ea merafo ea barium, ho qhibiliha le ho etsa tlhahiso. Ho phaella moo, barium le metsoako ea eona e sebelisoa haholo. Matsoai a tloaelehileng a chefo a barium a kenyelletsa barium carbonate, barium chloride, barium sulfide, barium nitrate, le barium oxide. Litlhoko tse ling tsa letsatsi le letsatsi li boetse li na le barium, tse kang barium sulfide lithethefatsing tse tlosang moriri. Likokoana-hloko tse ling tsa temo le li-rodenticides li na le letsoai le qhibilihang la barium joalo ka barium chloride le barium carbonate.
Nako ea poso: Jan-15-2025